The key difference between acid and base is that acids are substances that release hydrogen ions (H+) in solution, while bases are substances that accept hydrogen ions or release hydroxide ions (OH-) in solution.
What is Acid?
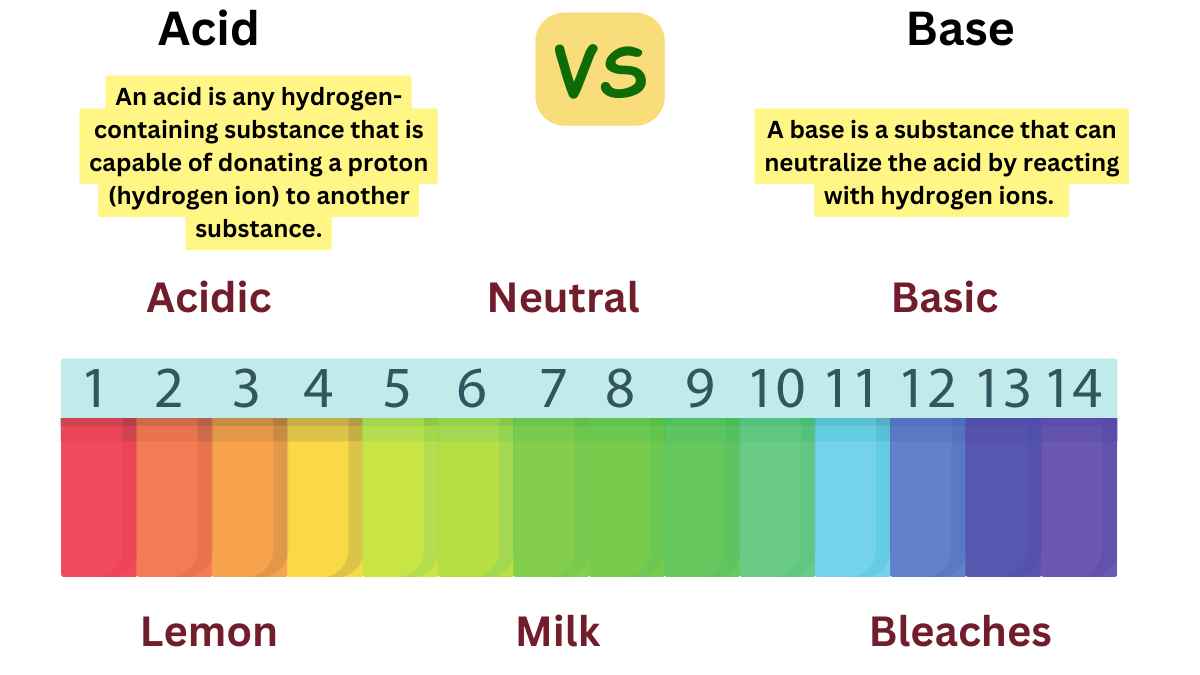
An acid is a type of chemical substance that, when dissolved in water, donates protons (H⁺ ions) to the solution. Acids are characterized by their sour taste, ability to turn blue litmus paper red, and their pH value of less than 7. They are also known for their ability to react with metals to produce hydrogen gas and to react with bases to form salts and water.
Acids can be classified into two main categories:
Strong Acids
These acids completely dissociate into ions when dissolved in water, releasing a high concentration of H⁺ ions. Examples include hydrochloric acid (HCl) and sulfuric acid (H₂SO₄).
Weak Acids
These acids only partially dissociate into ions when dissolved in water, resulting in a lower concentration of H⁺ ions. Examples include acetic acid (found in vinegar) and citric acid (found in citrus fruits).
Acids play crucial roles in various chemical reactions and processes in both nature and industry. They are essential components in areas such as digestion (stomach acid), chemical synthesis, and industrial processes.
What is Base?
A base, in the context of chemistry, is a substance that can accept protons (H⁺ ions) or donate pairs of electrons in chemical reactions. Bases are characterized by their bitter taste, slippery feel, and the ability to turn red litmus paper blue. They generally have a pH value greater than 7 in aqueous solutions.
Bases can be categorized into two main types:
Arrhenius Bases
These are substances that, when dissolved in water, release hydroxide ions (OH⁻). The presence of hydroxide ions in a solution makes it basic.
Bronsted-Lowry Bases
According to the Bronsted-Lowry theory of acids and bases, a base is defined as a substance capable of accepting a proton (H⁺ ion) in a chemical reaction. This theory allows for a broader understanding of bases, as they can interact with acidic substances without necessarily involving water.
Common examples of bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), ammonia (NH₃), and magnesium hydroxide (Mg(OH)₂). Bases have a variety of applications, ranging from household cleaning products to industrial processes and chemical reactions.
Acid vs Base
The major difference between acid and base is given below:
| Property | Acids | Bases |
| Taste and Touch | Sour taste. | Bitter taste, slippery to touch. |
| pH | pH less than 7. | pH greater than 7. |
| Ionization in Water | Release H⁺ ions (protons). | Release OH⁻ ions (hydroxides). |
| Electrolyte Conductivity | Conduct electricity in solution. | Conduct electricity in solution. |
| Reaction with Metals | React with active metals to produce hydrogen gas. | Typically do not react with metals. |
| Indicators | Turn blue litmus paper red. | Turn red litmus paper blue. |
| Chemical Properties | Donate protons (H⁺ ions) in reactions. | Accept protons (H⁺ ions) in reactions. |
| Common Examples | Hydrochloric acid (HCl), sulfuric acid (H₂SO₄). | Sodium hydroxide (NaOH), ammonia (NH₃). |
