The key difference between aldehydes and ketones is that aldehydes have a terminal carbonyl group (CHO) while ketones have an internal carbonyl group (C=O).
What Are Aldehydes?
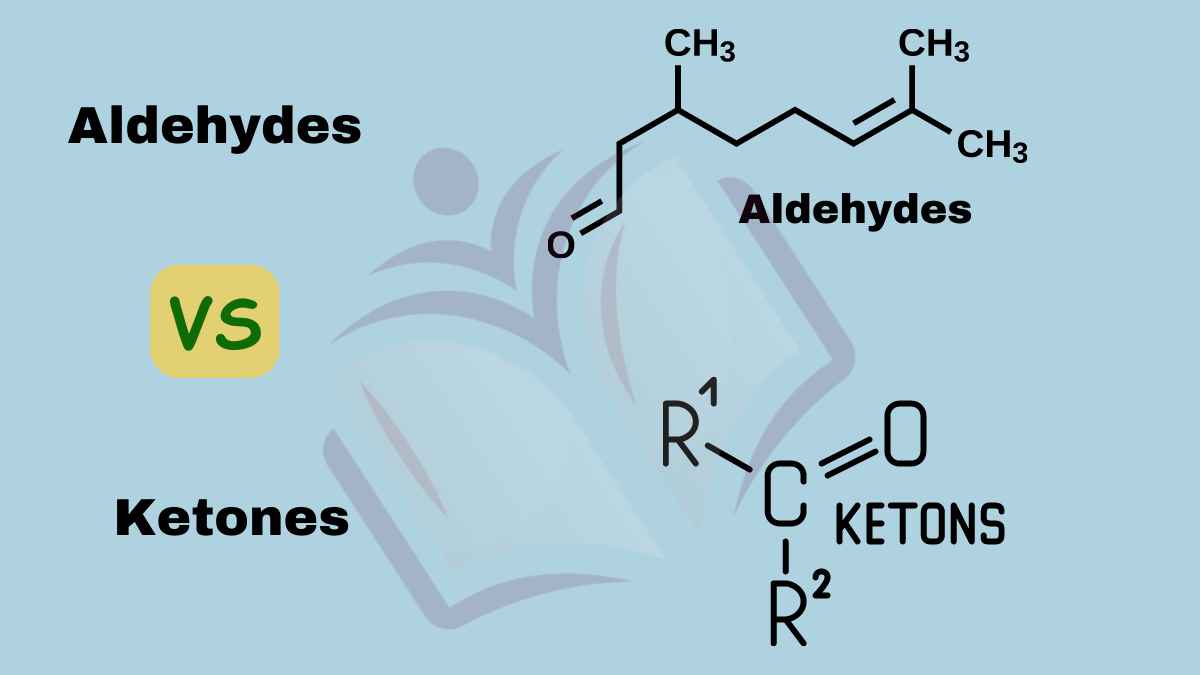
Aldehydes are a class of organic compounds characterized by a functional group called the aldehyde group, consisting of a carbonyl group (C=O) bonded to a hydrogen atom (H). They are commonly found in various natural and synthetic substances, including fragrances, flavors, and pharmaceuticals.
Aldehydes play crucial roles in chemical reactions as both reactants and intermediates. They are often volatile and possess distinctive odors, contributing to the aroma of essential oils and perfumes. Aldehydes can undergo oxidation to form carboxylic acids and can be reduced to primary alcohols. Their diverse reactivity and applications make them essential in both industrial and biological contexts.
What Are Ketones?
Ketones are organic compounds characterized by a carbonyl group (C=O) attached to two carbon atoms within the molecule. This functional group is situated at the center of the carbon chain, distinguishing ketones from other compounds like aldehydes and carboxylic acids.
Ketones play vital roles in various biological and chemical processes. They are commonly found in nature, often as intermediate products in metabolism and energy production. Ketones can be synthesized through controlled oxidation of secondary alcohols or by the breakdown of fatty acids. They also serve as important precursors in the synthesis of pharmaceuticals, flavors, and fragrances due to their versatile reactivity and structural diversity.
Aldehydes Vs Ketones
The primary difference between aldehydes and ketones is given below:
| Characteristic | Aldehydes | Ketones |
| Functional Group | -CHO (carbonyl group at the end of a carbon chain). | -C(O)R (carbonyl group between two carbon chains). |
| Naming Suffix | -al | -one |
| General Formula | RCHO | RCOR’ |
| Position of Carbonyl Group | At the end of the carbon chain. | Between carbon atoms. |
| Boiling Point | Generally lower than corresponding ketones. | Generally higher than corresponding aldehydes. |
| Odor | Often have strong and distinctive odors. | Generally milder odor compared to aldehydes. |
| Reactivity | More prone to oxidation reactions. | Less prone to oxidation reactions. |
| Prevalence | Often found in naturally occurring compounds, e.g., essential oils. | Common in a variety of organic compounds, such as pharmaceuticals and solvents. |
| Examples | Formaldehyde, Acetaldehyde. | Acetone, Benzophenone. |
