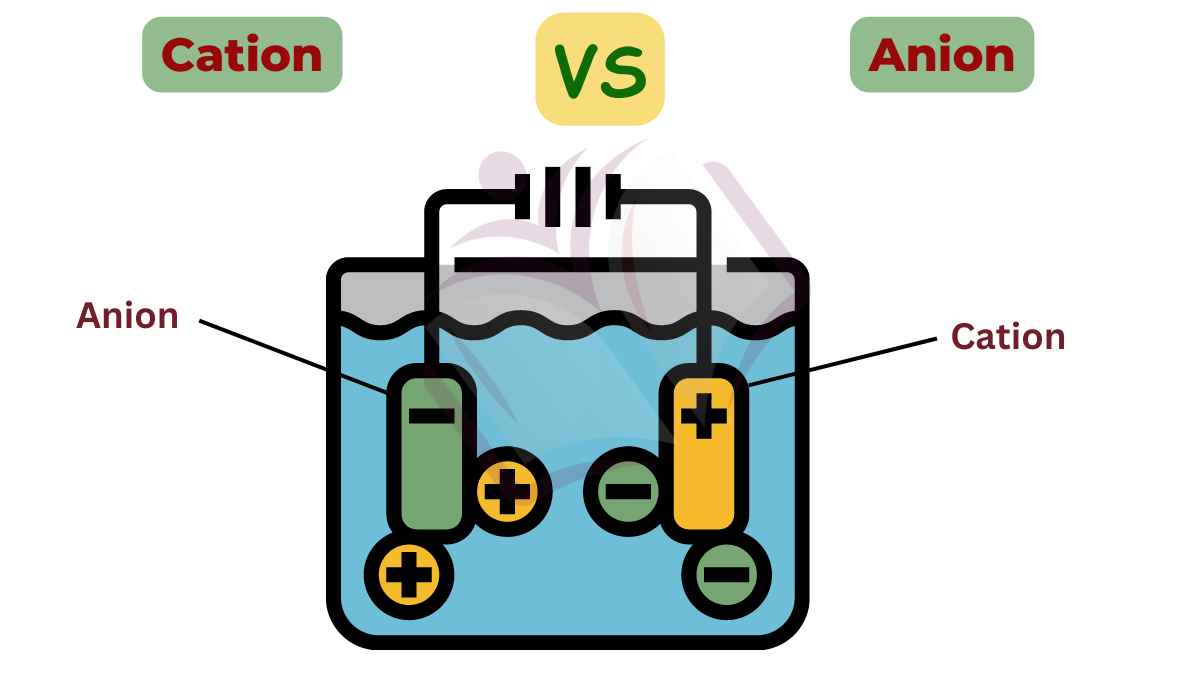
The key difference between cation and anion is that cations are positively charged ions, while anions are negatively charged ions.
What is Cation?
A cation is an ion that carries a positive electric charge due to the loss of one or more electrons from its outer shell. Cations are formed when atoms of elements undergo a process called ionization, during which they lose electrons and become positively charged. cations play a crucial role in various chemical reactions and are often involved in forming chemical bonds with anions (negatively charged ions) to create stable compounds.
The number of protons in a cation’s nucleus remains the same as its parent atom, but the number of electrons decreases, leading to an overall positive charge. Cations are attracted to negatively charged regions or particles, such as anions or the cathode in an electrical circuit.
Some common examples of cations include Na+ (sodium ion), Ca2+ (calcium ion), and Al3+ (aluminum ion). Cations are important in biological systems, as they regulate the movement of ions across cell membranes and influence nerve impulse transmission.
In analytical chemistry, cations can be identified and quantified using various techniques, such as ion chromatography or mass spectrometry. The charge and concentration of cations in soil influence soil fertility, affecting plant growth and agriculture.
What is Anion?
An anion is a type of ion, which is an electrically charged particle formed when an atom gains one or more electrons. When an atom gains electrons, it becomes negatively charged, and this negatively charged species is called an anion. Anions are essential in various chemical processes and play a crucial role in forming ionic compounds.
The negative charge of anions results from having more electrons than protons, creating an overall negative electrical charge. Anions can be monoatomic (e.g., chloride, Cl-) or polyatomic (e.g., sulfate, SO4^2-) depending on whether they consist of a single atom or a group of atoms bonded together. In nature, anions are present in various compounds, including salts, acids, and bases, significantly influencing the properties of these substances.
Anions are often involved in important biological processes, such as ion transport across cell membranes and enzyme functions. They are attracted to the positively charged electrode (anode) during electrolysis. Anions are named using specific rules, typically ending with “-ide” for monoatomic anions (e.g., fluoride) and other suffixes for polyatomic anions (e.g., nitrate, carbonate).
Cation Vs Anion
The major difference between cation and anion are enlisted below:
| Characteristic | Cation | Anion |
| Charge | Positive | Negative |
| Formation | Formed by losing electrons. | Formed by gaining electrons. |
| Ionization | Result from the loss of electrons from an atom. | Result from the gain of electrons by an atom. |
| Examples | Na+ (sodium ion), Ca2+ (calcium ion). | Cl- (chloride ion), O2- (oxide ion). |
| Size | Smaller than the neutral atom. | Larger than the neutral atom. |
| Common Sources | Metals and metal-like elements. | Attracts toward positively charged ions and molecules. |
| Electrostatic Attraction | Attracts toward negatively charged ions and molecules. | Attracts toward negatively charged ions and molecules. |
| Role in Compounds | Often found in ionic compounds, which are typically salts. | Often found in ionic compounds, forming compounds like oxides and acids. |
