The key difference between galvanic and electrolytic cell is that galvanic cells spontaneously convert chemical energy into electrical energy, while electrolytic cells use electrical energy to drive non-spontaneous chemical reactions.
What is a galvanic cell?
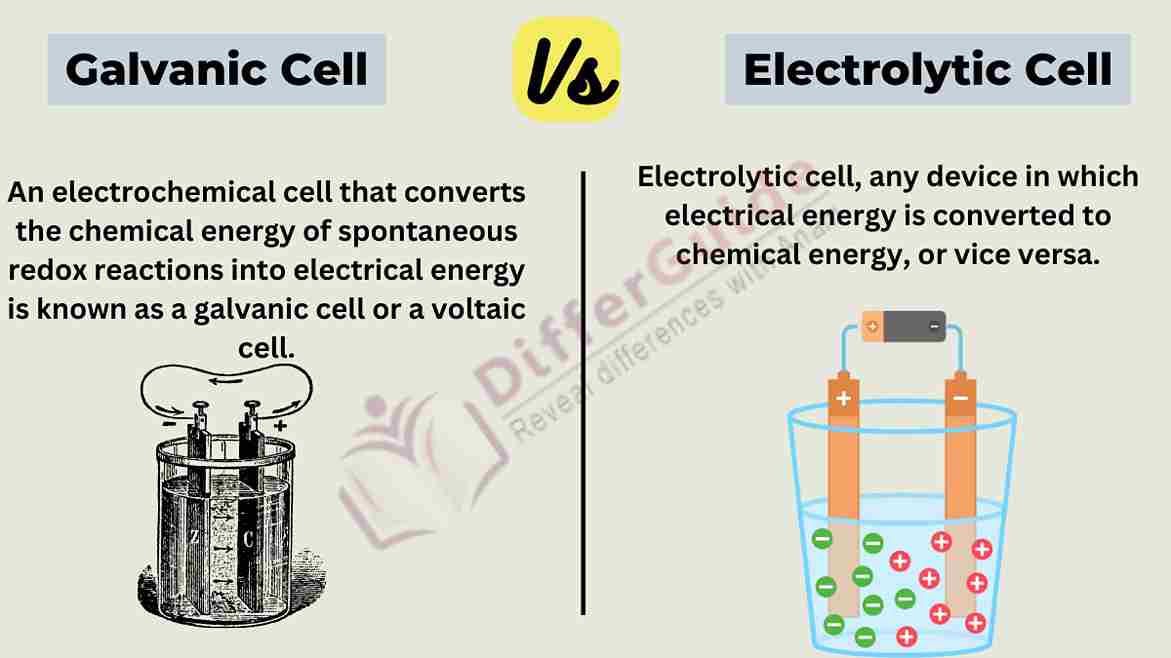
A galvanic cell, also known as a voltaic cell, is an electrochemical device that converts chemical energy into electrical energy through a spontaneous redox (reduction-oxidation) reaction. This type of cell generates an electric current as a result of the natural tendency of certain chemical reactions to transfer electrons from one substance to another.
In a galvanic cell, two half-cells are connected by a conductive pathway, often a wire. Each half-cell consists of an electrode (a solid conductor) immersed in an electrolyte solution (a liquid containing ions). One electrode serves as the site of oxidation, where a substance loses electrons (anode), while the other electrode serves as the site of reduction, where a substance gains electrons (cathode).
During the redox reaction, electrons flow from the anode to the cathode through the external circuit, generating an electric current. The movement of electrons through the wire constitutes useful electrical energy that can be harnessed for various applications.
Examples of galvanic cells include common batteries like alkaline batteries, lead-acid batteries used in automobiles, and even biological systems like nerve cells and certain types of bacterial metabolism.
What is an Electrolytic Cell?
An electrolytic cell is an electrochemical device that uses electrical energy to drive a non-spontaneous chemical reaction. Unlike the galvanic (voltaic) cells, which produce electrical energy from spontaneous chemical reactions, electrolytic cells require an external source of electrical energy to facilitate a chemical reaction that wouldn’t occur naturally.
In an electrolytic cell, an external voltage is applied across two electrodes immersed in an electrolyte solution. The electrodes are typically made of conductive materials like metals or graphite. The electrolyte contains ions that can move and participate in the electrochemical reaction.
When the external voltage is applied, it causes electrons to flow from the positive electrode (anode) to the negative electrode (cathode). At the anode, oxidation reactions take place, releasing electrons into the circuit. At the cathode, reduction reactions occur, where electrons from the circuit reduce ions from the electrolyte.
Electrolytic cells are used for various applications, including electroplating (depositing a layer of metal onto a surface), metal refining, electrolytic production of chemicals, and even in processes like water electrolysis to produce hydrogen and oxygen gases.
Galvanic vs Electrolytic cell
The main difference between galvanic and electrolytic cells is given below:
| Galvanic (Voltaic) Cell | Electrolytic Cell | |
| Energy Conversion | Converts chemical energy to electrical energy. | Converts electrical energy to chemical energy. |
| Spontaneity | Spontaneous (generates its own electrical energy). | Non-spontaneous (requires external electrical energy). |
| Anode and Cathode | Anode is the site of oxidation (loses electrons). | Anode is the site of oxidation (loses electrons). |
| Electrons flow from the anode to cathode. | The cathode is the site of reduction (gains electrons). | |
| Electron Flow | Electrons flow from the anode to the cathode. | Electroplating, metal refining, and electrolytic production of chemicals. |
| Salt Bridge/ | A salt bridge or porous barrier is used to maintain ion balance and allow ion flow. | An electrolyte is used to maintain ion balance and allow ion flow. |
| Porous Barrier | between half-cells. | within the electrolyte. |
| Electrolyte | Electrolyte solutions in half-cells are connected by a salt bridge. | The redox reaction is forced by applying an external potential difference. |
| Purpose | Used to generate electrical energy. | Used in electroplating, electrolysis, and other chemical processes. |
| Example Applications | Batteries, fuel cells. | The electrolyte solution is present within the cell itself. |
| Reaction | Redox reaction occurs spontaneously, producing a potential difference. | Electrons flow from the cathode to the anode. |
